Author: Eugene Lukhtanov, Noah Scarr, Mark Gleave, Eric Yau
ELITechGroup MDx LLC, 21720 23rd Dr SE, Suite 150, Bothell, WA 98021
Advantages of AquaPhluor® 639
ELITechGroup (formerly Epoch Biosciences) has been developing improved fluorescent dyes for oligonucleotide labeling for over two decades. When in their labeling reagent form, AquaPhluor® dyes, one of our fluorophore lines, possess latent phosphonate groups in the linkers. Fully protected and neutral, these groups are compatible with phosphoramidite preparation and solid phase synthesis. In their ultimate form, upon completion of standard oligonucleotide synthesis, the dyes are negatively charged and thus significantly more hydrophilic. The phosphonate chemistry of AquaPhluor® dyes makes it possible to combine the versatility of automated oligonucleotide synthesis with the benefits of hydrophilicity. One particularly attractive AquaPhluor® dye is AquaPhluor® 639 (AP639).
| A., | B. | ||||||||||
 |
|
||||||||||
| C. | D. | ||||||||||
 |
 |
||||||||||
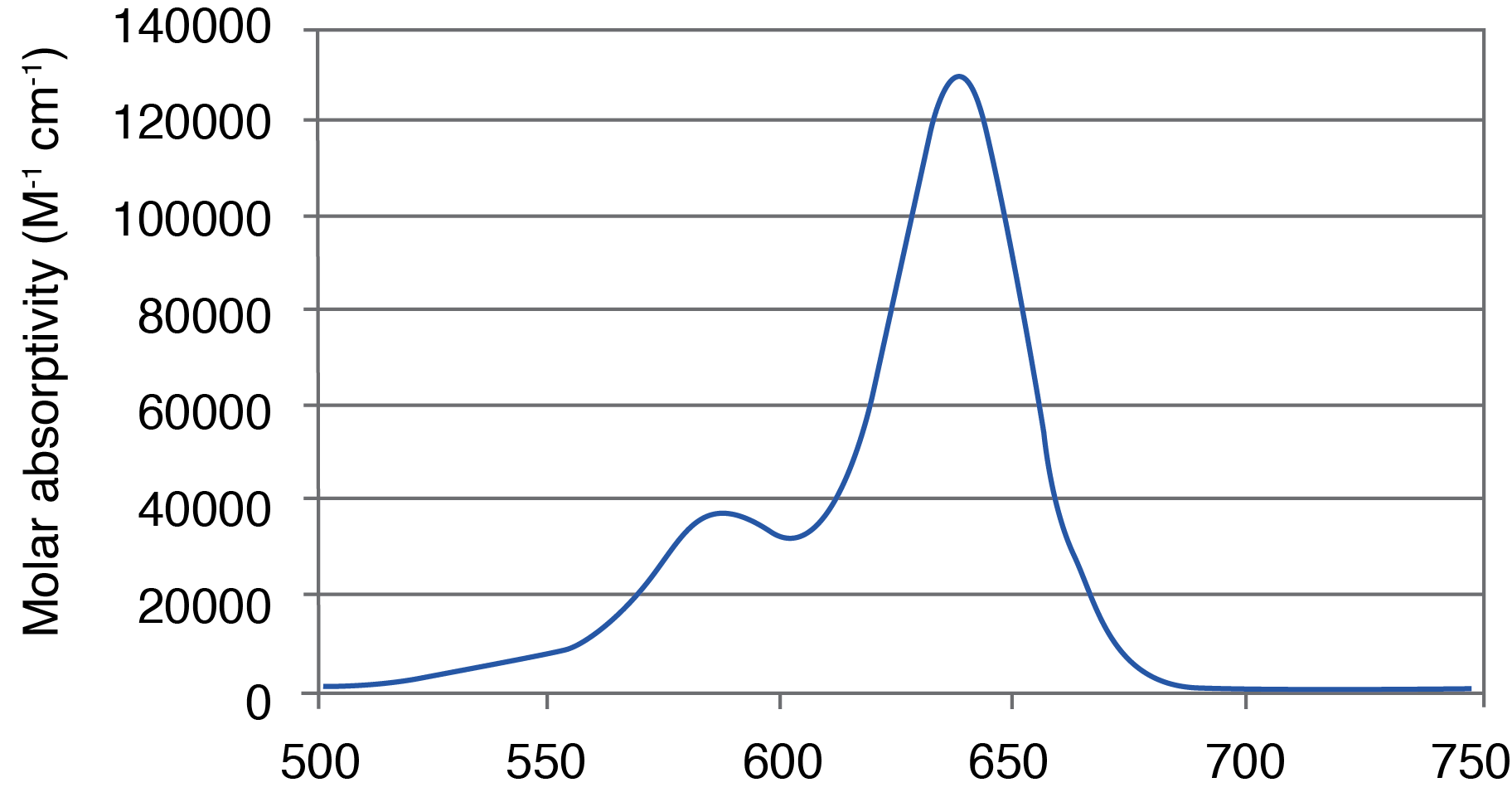
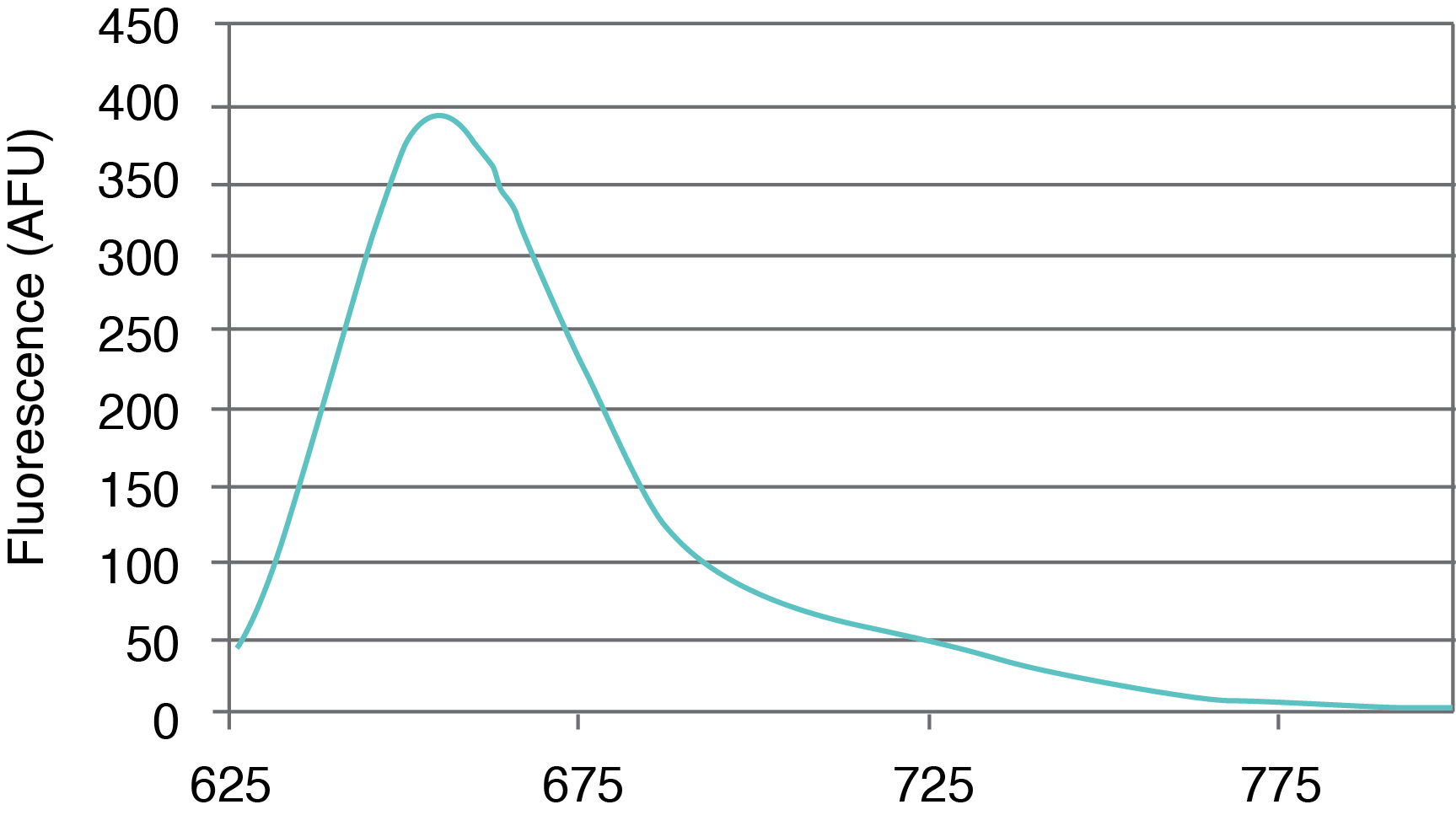
Figure 1. AquaPhluor® 639. A, AP639 chemical structure; B, AP639 spectral properties; C, AP639 molar absorptivity; D, AP639 fluorescence.
|
|||||||||||
AP639 is a red-shifted rhodamine dye that exhibits many desirable properties (Figure 1). The dye is multiply charged with a net charge of -1, and with absorbance and extinction maxima at 639 and 655 nm, respectively. AP639 is relatively bright, with an extinction coefficient of 129,300 M-1cm-1 (639 nm) and a quantum yield of 0.28 (20 °C). In addition to sequence- and pH-independent (pH 5-8) fluorescence, AP639 also shows excellent chemical and photochemical stability, as well as temperature-independent fluorescence. These last three properties make AP639 an attractive alternative to Cyanine 5 and other similarly-structured Cyanine analogues in the red region of the visible spectrum.
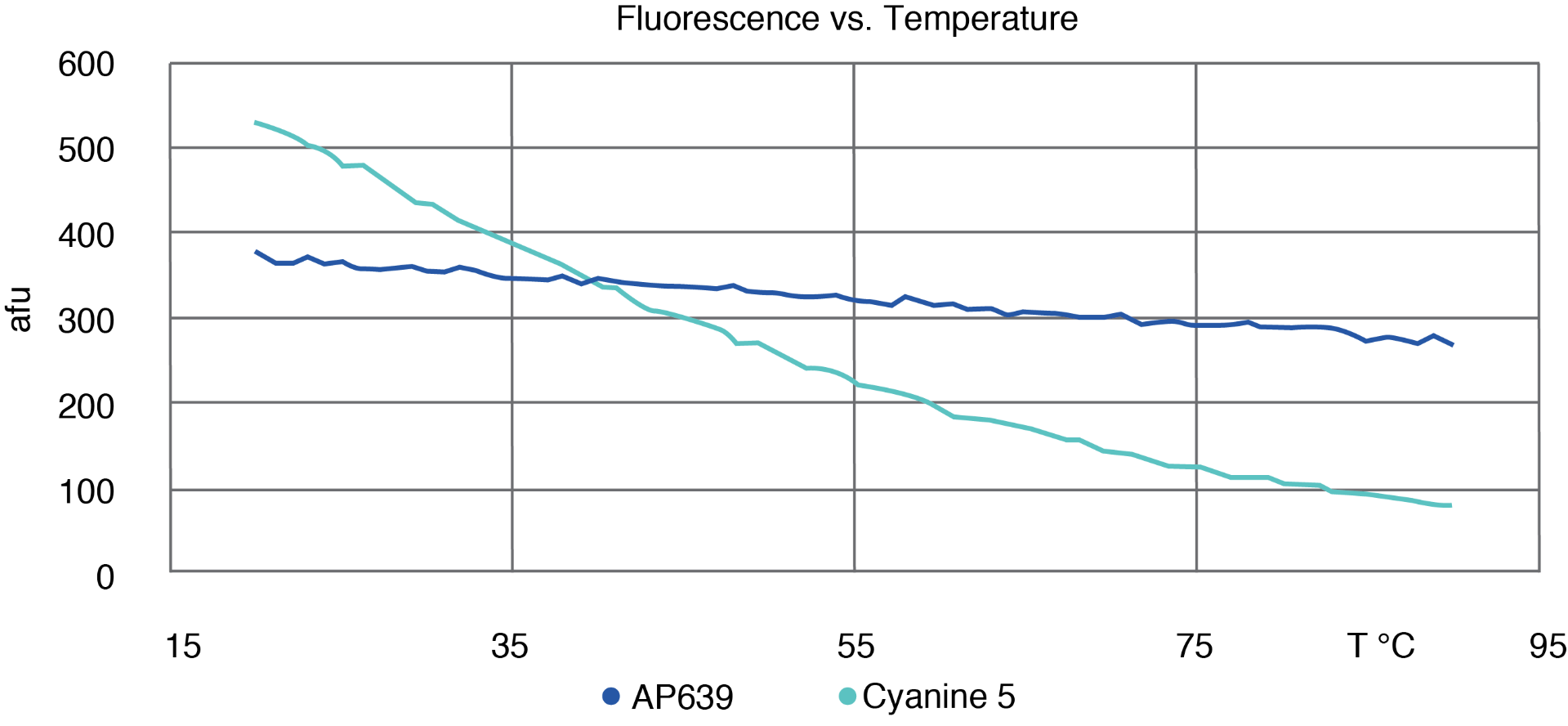
AP639 differs from Cyanine 5 in three key aspects. Firstly, it displays relatively temperature-independent fluorescence that only decreases a modest 20 % when the temperature is increased from 20 to 80 °C (Figure 2). This is a stark contrast to Cyanine 5, which loses 80 % of its fluorescence intensity over the same temperature range. This difference allows AP639 to be particularly effective in higher temperature applications such as PCR, in which AP639 is at least twice as bright as Cyanine 5 under typical elongation conditions. AP639 is also very resistant to photobleaching. In one study, we monitored the fluorescence of AP639 and Cyanine 5 under constant exposure to visible light over time (Figure 3). Over a period of 2 hours, AP639 fluorescence was essentially unchanged, whereas Cyanine 5 fluorescence was reduced by about 50 %. This stability is particularly relevant for applications involving high intensity illumination, such as confocal microscopy. Finally, AP639 is much more stable to deprotection conditions. Unlike Cyanine 5, AP639 is stable to standard ammonium hydroxide conditions with <5 % of decomposition or side reactions. This allows for improved synthesis yields, as well as for fewer restrictions during oligonucleotide manufacturing.

To illustrate the utility of AP639, a serially diluted set of target DNA was amplified by qPCR on an ABI7500 Fast Dx system, using a set of primers and an MGB TaqMan probe labeled either with AP639 or Cyanine 5 at the 5’-end (Figure 4). Clear signal improvement was observed for the AP639-labeled probe, with a final amplification fluorescence close to three times higher than that of the Cyanine 5-labeled probe. This difference can be especially important for the amplification and detection of low target samples, which require more amplification cycles when signals tend to drop in intensity.

In summary, the new AquaPhluor® 639 dye offers numerous chemical stability and performance improvements over the traditional Cyanine 5. Over the years, Glen Research has brought several of our products to a wider customer base, and we hope that their customers will be able to realize the strengths of AP639 in fluorescence-based applications.